
Edwards Lifesciences has announced the results of a health economics study across nine European countries, led by York Health Economics Consortium, which it says has shown that early transcatheter aortic valve implantation (TAVI) for asymptomatic severe aortic stenosis (AS) patients delivers significant economic benefits alongside improved clinical outcomes.
The simulation estimates lifetime per-patient savings between £1,788 in the UK and CHF 15,802 in Switzerland, mainly driven by fewer strokes and hospitalisations compared to the traditional ‘watchful waiting’ approach.
Presented at PCR London Valves 2025 (16–18 November, London, UK), Edwards says that this is the first cost-effectiveness analysis of prompt treatment with TAVI in Europe. It is based on data from the EARLY TAVR trial using Edwards’ Sapien 3 TAVI platform.
The findings support updated European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines which give a class IIa, level of evidence A recommendation for earlier intervention in asymptomatic severe AS patients, the company adds.
“These new data reinforce the need to move away from a ‘wait-and-see’ approach for patients with asymptomatic severe AS. In Europe, approximately 100,000 patients suffer from asymptomatic severe AS every year. Treating patients earlier—before symptoms develop—not only improves clinical outcomes but also optimises healthcare costs and resource utilisation,” said Philippe Généreux (Morristown Medical Center, Morristown, USA). “AS patients, especially when asymptomatic, often go undiagnosed and untreated, leading to increased morbidity and mortality. This analysis underscores the importance of screening and detection strategies to improve referral pathways and patient outcomes, while reducing healthcare costs.”
Edwards Lifesciences’ Sapien 3 has been the only platform approved for treating asymptomatic severe AS since receiving CE mark extension in July 2025.
At TCT 2025, seven-year results from the PARTNER 3 were released, demonstrating the long-term performance of the Sapien platform.
“This new health economics analysis together with the latest ESC/EACTS guidelines confirm that prompt intervention delivers lasting value for all: better outcomes and quality of life for patients, a more proactive approach to disease management for clinicians, and long term savings for healthcare systems.” said Annette Brüls, corporate vice president EMEA, Canada, and Latin America, Edwards Lifesciences. “Combined with the long term clinical data presented at TCT 2025, current evidence strongly reinforces the benefits of TAVR and its proven durability.”


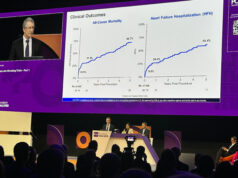








This analysis makes a compelling case for rethinking the traditional “watchful waiting” approach in asymptomatic severe aortic stenosis. Early TAVI not only improves patient outcomes but also appears to offer meaningful cost savings across diverse healthcare systems. What stands out is how economic modeling aligns with clinical evidence, reinforcing that timely intervention can be both life-saving and resource-efficient. It will be interesting to see how these findings influence adoption in countries where asymptomatic patients are often managed conservatively.