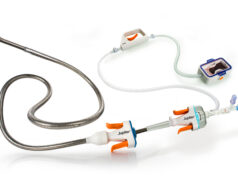
Vesalio has announced US Food and Drug Administration (FDA) 510(k) clearance and the upcoming US commercial launch of enVast, a clot retriever specifically cleared for mechanical thrombectomy in the cardiac circulation.
Vesalio has announced US Food and Drug Administration (FDA) 510(k) clearance and the upcoming US commercial launch of enVast, a clot retriever specifically cleared for mechanical thrombectomy in the cardiac circulation.
Large thrombus burden (LTB) is a common and challenging finding in patients undergoing primary percutaneous coronary intervention (PCI). Prompt and effective restoration of coronary flow is critical to minimising myocardial damage, reducing procedural complications, and improving clinical outcomes, the company says in a press release.
Powered by Vesalio’s proprietary Drop Zone technology, enVast delivers is capable of removing the full spectrum of coronary thrombi, including soft, fragment-prone clots, as well as dense, fibrin-rich thrombi that may be resistant to existing aspiration or retrieval techniques.
enVast is the first Vesalio product platform to receive regulatory clearance in both the USA as well as in Europe.
“With FDA clearance and upcoming US launch of enVast, we are proud to introduce a device that we truly believe redefines coronary thrombectomy,” said Steve Rybka, chief executive officer of Vesalio. “enVast is the first coronary-specific retriever designed with a stent-based clot capture architecture enhanced by our proprietary Drop Zone technology. Clinical experience internationally has consistently demonstrated its safety and effectiveness in managing complex LTB situations. We’re excited to now make this impactful solution available to US interventional cardiologists and their patients.”