 The US Centres for Medicare & Medicaid Services (CMS) has issued its proposed National Coverage Determination (NCD) on renal denervation, recommending coverage for the therapy for the treatment of uncontrolled hypertension.
The US Centres for Medicare & Medicaid Services (CMS) has issued its proposed National Coverage Determination (NCD) on renal denervation, recommending coverage for the therapy for the treatment of uncontrolled hypertension.
A 30-day public comment period is pen for healthcare professionals, professional societies, and industry to provide input before CMS issues its final determination in October.
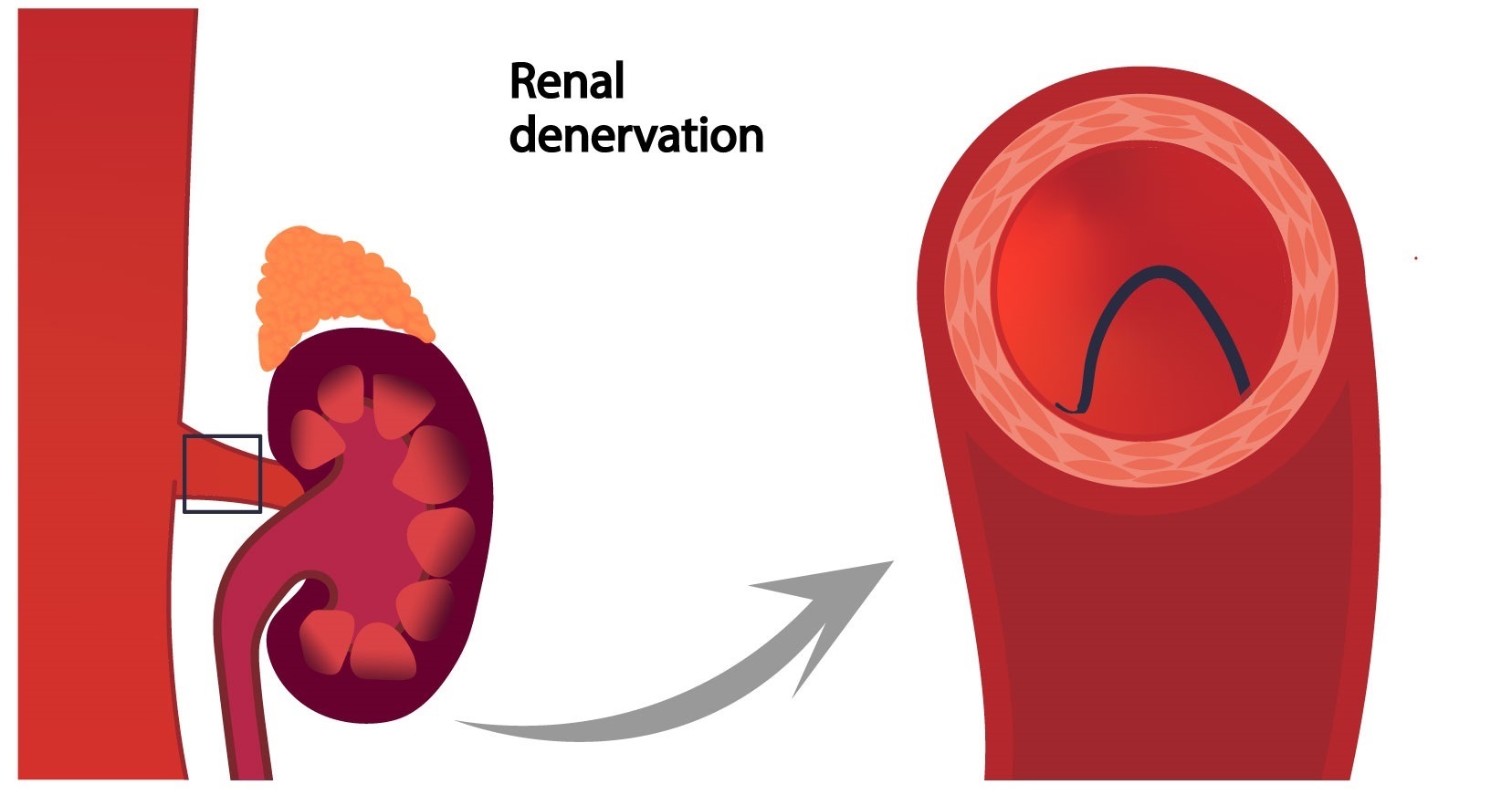
Renal denervation is a treatment for individuals whose blood pressure cannot be managed with lifestyle changes and medication, in which energy is targeted through a catheter to the renal nerves to modulate the sympathetic signalling between the kidneys and brain to reduce blood pressure.
Two devices have been approved by the US Food and Drug Administration (FDA)—the Paradise (Recor Medical) and Symplicity Spyral (Medtronic) systems—following a review of evidence from trials assessing both technologies. Medtronic‘s Symplicity Spyral system is a minimally invasive procedure that delivers radiofrequency energy to nerves near the kidneys that can become overactive and contribute to high blood pressure, whilst the Paradise system is an ultrasound-based technology.
CMS opened its National Coverage Analysis (NCA) on the therapy in January 2025, after having granted granted transitional pass-through (TPT) payment for the two systems in late 2024. Both companies have issued statements welcoming CMS’ latest step.
“We are encouraged by CMS’s proposed decision to provide Medicare coverage for uRDN [ultrasound renal denervation] in patients with uncontrolled hypertension—a population that continues to face significant unmet need,” said Lara Barghout, chief executive officer of Recor Medical. “This preliminary determination is a meaningful step forward in recognising the clinical value of renal denervaton and will aid in expanding access to the patients who need it.”
“We are pleased to see CMS’ proposed NCD for the Symplicity Spyral renal denervation system,” said Jason Weidman, senior vice president and president of the Coronary and Renal Denervation business, which is part of the Cardiovascular Portfolio at Medtronic. “This milestone is an important step towards expanding patient access to this innovative treatment for hypertension, a public health crisis in the USA.
“The proposed NCD from CMS—as it stands today—will provide many US Medicare patients in need with access to renal denervation. We recognise and thank CMS for their ongoing efforts in creating new pathways to expedite access to breakthrough technologies.”










