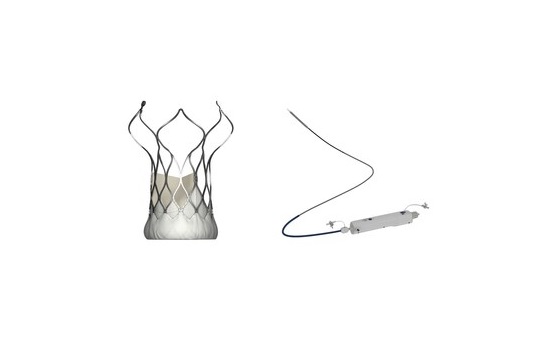
 MicroPort CardioFlow Medtech Corporation has announced that its self-developed second-generation transcatheter aortic valve implantation (TAVI) device, the VitaFlow Liberty transcatheter aortic valve and retrievable delivery system has received EU CE-MDR certification.
MicroPort CardioFlow Medtech Corporation has announced that its self-developed second-generation transcatheter aortic valve implantation (TAVI) device, the VitaFlow Liberty transcatheter aortic valve and retrievable delivery system has received EU CE-MDR certification.
The VitaFlow series TAVI solution along with its accessory—the Alwide series balloon catheter—has successfully covered nearly 700 core hospitals in 10 countries and regions, treating more than 10,000 patients with aortic valve disease worldwide.
The clinical data from VitaFlow series valves were revealed at PCR London Valves 2023 (19–21 November, London, UK) highlighting the device’s long-term clinical performance aligning with international standards.
The long-term results of VitaFlow in high surgical risk patients with severe aortic stenosis showed promising outcomes in all-cause mortality, cardiac mortality, and permanent pacemaker implantation rates for patients over seven years, compared to other similar studies.
During the conference, Darren Mylotte (Galway University Hospitals, Galway, Ireland), commented on the excellent data, and introduced the advantages of VitaFlow Liberty in its motorised delivery system.
The system can assist the valve to position easily due to its flexibility and 360° range of motion when treating complex anatomical patients with severe angled aortic arch deformities. The valve can also be fully retrieved and repositioned when released to 75%, and provides up to three retrievable opportunities for each procedure.
Before launching into the EU market, VitaFlow Liberty conducted premarket clinical implantations at Galway University Hospital in Ireland, Rigshospitalet (Copenhagen University Hospital) in Denmark, and St Thomas’ Hospital as well as Brighton & Sussex University Hospitals NHS Trust in the UK, and received very high appraisal from many well-known clinical professionals.
Ole De Backer, a professor of interventional cardiology, who led the TAVI procedures at Rigshospitalet, said: “The overall release process of VitaFlow Liberty is notably stable, ensuring precise positioning. This stability is especially crucial in patients with small left ventricles, where VitaFlow Liberty consistently achieves stable and precise deployment, fully demonstrating its distinct advantages. We look forward to its positive impact on a broader patient population following CE certification.”
It has been reported that the European post-market clinical project will also be planned to start this year.
As part of CardioFlow’s global expansion roadmap, the company has also achieved milestones with CE application on three of its products, including the Alwide Plus balloon catheter, the AnchorMan left atrial appendage closure system and the AnchorMan left atrial appendage access system, both developed by its subsidiary, CardioAdvent.
Jeff Lindstrom, president of CardioFlow, said: “The certification of VitaFlow Liberty by the CE regulatory body under MDR is a testament to CardioFlow’s world class R&D, quality, and clinical capabilities. This recognition will expedite the global clinical adoption of the VitaFlow series along with other innovative products, advancing CardioFlow’s globalisation strategy. This achievement also positions us to make a more substantial contributions to developments in the field of heart valve interventions, ultimately benefiting patients across the globe.”
Guoming Chen, chairman of CardioFlow, commented: “Securing the EU CE-MDR marking for VitaFlow Liberty is not just a passport for the product’s entry into the European market, it also represents a significant milestone in CardioFlow’s history and global roadmap. This achievement will assist in diversifying the company’s sources of sales revenue and bolstering our overall competitiveness with a steadfast commitment to world class product innovation.”










