Admedus has announced that, in collaboration with its regional partner Genpharm, it has received market approval for CardioCel in the United Arab Emirates (UAE) and first orders. This follows on from the recent approval of the product in Kuwait.
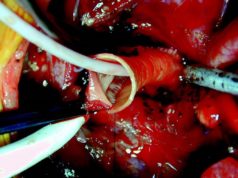
A press release notes that CardioCel is a single-ply bioscaffold that remains functional, durable and free from calcification. It adds that engineered with Admedus’ proprietary Adapt technology, CardioCel provides superior restorative structural heart repair and reconstruction. Long-term clinical studies using CardioCel have shown no calcification eight years’ post-implantation. It is used in leading heart centres around the world for cardiovascular repairs and reconstructions.
Admedus chair and interim chief executive officer, Wayne Paterson, says: “As we continue to drive strong sales growth of CardioCel, this approval is a significant milestone in terms of our expansion of the product franchise across emerging markets. The UAE is a strategically important location as a central hub in Middle East and North Africa for performing cardiovascular surgery.”