 Cardiac Dimensions has announced that Australia’s Therapeutic Goods Administration (TGA) has approved its Carillon mitral contour system, a right heart transcatheter mitral valve repair (TMVr) device. The device is designed to treat the primary cause of functional mitral regurgitation (FMR) in patients with mitral regurgitation grades 2+, 3+ and 4+.
Cardiac Dimensions has announced that Australia’s Therapeutic Goods Administration (TGA) has approved its Carillon mitral contour system, a right heart transcatheter mitral valve repair (TMVr) device. The device is designed to treat the primary cause of functional mitral regurgitation (FMR) in patients with mitral regurgitation grades 2+, 3+ and 4+.
“We would like to thank the Therapeutic Goods Administration for their partnership in the review and approval of the Carillon System in Australia,” said Rick Wypych, chief executive officer and President of Cardiac Dimensions. “This is a significant milestone as Australian patients with heart failure can now be treated earlier in their disease state with a minimally invasive treatment for mitral regurgitation.”
“The availability of the Carillon System is an extremely exciting advancement for the medical community as this technology has the potential to help millions of patients suffering from heart failure and FMR,” commented David Kaye, director of cardiology at The Alfred Hospital, Melbourne, Australia. “The Carillon System provides a new, non-surgical method to correct a fundamental problem with the mitral valve in patients with heart failure. This approach has been shown to reduce mitral regurgitation and to favourably impact the left ventricle (LV). This is significant because a reduction in LV enlargement in heart failure is known to be associated with positive patient outcomes.”
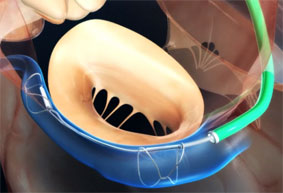
The Carillon System is placed using a catheter-based technique in a vein on the outside of the heart that is adjacent to the mitral valve. This procedure is designed to reshape the mitral valve, reduce valve leakage, and thus reduce mitral regurgitation and induce favourable remodelling.
The TGA’s approval was based on the combined efficacy and safety results from several Cardiac Dimensions’ studies including the most recent REDUCE FMR study. Treatment with the Carillon Mitral Contour System in these studies consistently demonstrated a significant decrease in regurgitant volume and left ventricular volumes, the company said in a press release. These changes were associated with reduced heart failure symptoms and improved quality of life.