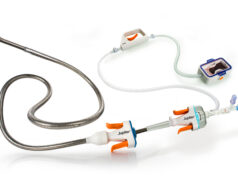
Aquapass has received Israeli marketing approval for its non-invasive system for fluid overload management in patients suffering from heart failure and chronic or end-stage kidney disease.
Aquapass has received Israeli marketing approval for its non-invasive system for fluid overload management in patients suffering from heart failure and chronic or end-stage kidney disease.
As part of the initial rollout, Aquapass will begin commercial deployment in selected medical centres across Israel, working with clinicians to collect clinical feedback and develop real-world evidence that will support broader adoption and long-term integration into patient care, the company says in a press release.
The ongoing US Food and Drug Administration (FDA) pivotal REFORM-HF trial, enrolling at hospitals in both the USA and Israel, continues. REFORM-HF is evaluating net fluid loss with and without the Aquapass system in patients with acutely decompensated heart failure who are not responding adequately to current medical treatment.
US sites include Cone Health (Greensboro), Rochester Regional Health (Rochester), University of Minnesota Medical Center (Minneapolis), Lenox Hill Hospital (New York), Austin Heart (Austin), and UCSD Medical Center (San Diego). The study is progressing, with enrolment completion targeted for Q1 2026 and submission for FDA clearance planned for Q2 2026. The approval covers a broad clinical indication across cardiology, nephrology, and general medicine, supporting use in both acute and chronic patient populations.
“This approval marks a pivotal transition for Aquapass—from bench to the bedside, and reinforces our commitment to serving patients worldwide,” said Noam Josephy, chief executive officer of Aquapass. “We are now a commercial-phase company with a clear mission: to serve the sickest acute and chronic patients. This achievement reflects the collective dedication, focus, and grit of our team, while putting patients first. We’ve taken a significant step toward transforming fluid overload management and therapy.”
Doron (Rambam Medical Center, Haifa, Israel), added: “Fluid overload is a common and serious problem for cardiac patients, often leading to repeated hospitalisations and deterioration in quality of life. The Aquapass system provides a much-needed, kidney-independent, non-invasive approach that has the potential to change how we manage these patients both in the hospital and in the community.”
Ron Wald (St Michael’s Hospital, Toronto, Canada, and Tel Aviv Sourasky Medical Center, Tel Aviv, Israel), commented: “The ability to safely and effectively remove excess fluid without relying on diuretics or ultrafiltration represents a breakthrough for patients with advanced chronic kidney disease and kidney failure. Aquapass could address a major gap in current clinical practice.”
Scott Feitell (Rochester Regional Health, Rochester, USA), principal investigator of REFORM-HF, said: “Patients hospitalised with acutely decompensated heart failure, often accompanied by worsening renal function, are among the most challenging we treat. A non-invasive, kidney-independent option to treat fluid overload could expand our toolkit, help decongest these patients more reliably, and potentially improve outcomes. REFORM-HF is designed to rigorously evaluate that promise.”