One-year outcomes from the early feasibility study (EFS) assessing AltaValve System (4C Medical Technolgies)—a supra-annular transcatheter mitral valve replacement (TMVR) system designed to overcome limitations of existing mitral replacement technologies—demonstrate sustained clinical benefits with the device, investigators have reported.
Presenting the latest results during a late-breaking trials session at PCR London Valves (16–18 November, London, UK), Vlasis Ninios (Interbalkan Medical Center, Thessaloniki, Greece) reported that, in a population of patients including many who would have been ineligible to receive other mitral valve technologies, implanters achieved a high rate of technical success with the procedure, resulting in symptomatic improvements for patients.
Left ventricular outflow tract (LVOT) obstruction is a common limitation of existing TMVR technologies leading to high rates of screen failure for patients screened for TMVR trials, Ninios highlighted in his presentation, ranking this alongside mitral annulus size and the presence of mitral annular calcification (MAC) as the exclusions that comprise the “Achilles’ heel” of existing TMVR technologies. AltaValve System’s atrial fixation TMVR device is designed to minimise the risk of LVOT obstruction and treat a broad population of mitral regurgitation (MR) patients as well as varied mitral annulus sizes.
The device is a transseptal TMVR platform whereby the prosthetic valve is positioned above the native mitral valve via an atrial-only fixation that is intended to ensure that cardiac structures are retained within the left ventricle. The AltaValve implant is also designed to allow future left atrial access for other procedures.
Ninios described the deployment and positioning of the device, which features a 27mm bovine pericardial valve encased in a nitinol stent frame, as a “straightforward, single-stage” procedure, whereby a steerable guide is inserted into the left atrium and flexed down towards the mitral valve, with the implant then deployed from the mitral annular level to the top of the left atrium. The valve is repositionable and recapturable throughout the procedure.
“We have seen in the EFS data that the anatomical acceptance rate in using this technology is 77%,” Ninios commented, adding that more than half (53%) of the 30 patients included in the trial had a narrow LVOT and had been rejected for other technologies. Added to this, 27% of the patients had large annuli, defined as a diameter >48mm, and moderate-to-severe MAC was seen in 23% of patients.
Enrolled at sites in Europe, USA and Japan, patients were eligible for inclusion if they were deemed high risk for surgery and had symptomatic, severe MR. More than half (63%) of patients were female, and there was an even split between patients with functional or degenerative MR.
The study included 13 patients who were treated via transapical approach, however today, only the less invasive transseptal approach is utilised. Seventeen patients in the study were treated using the transseptal approach.
Technical success of the procedure was high (97%) with only one patient converting to surgery. The device resulted in total elimination of MR in all cases, with mitral valve gradients averaging 2.5mmHg at baseline and 2.1mmHg post-procedure. Additionally, computed tomography (CT) analysis showed that the AltaValve implant increased the neo-LVOT by approximately 0.8cm2, and the valve’s annular ring moved away from the LVOT during systole, preserving native MV dynamics.
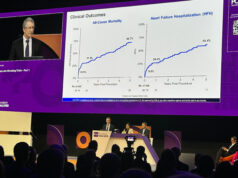
All-cause mortality stood at 17% at one year among patients in the transapical cohort and 7% in the transseptal cohort, with a rate of cardiac mortality of 14% among transapical patients and 0% among transseptal patients. Valve thrombosis occurred in one patient (who did not adhere to medication schedule), and one new pacemaker implantation was required across the entire cohort. There were no incidences of stroke, new-onset atrial fibrillation or need for mitral valve reintervention across the entire patient group. New York Heart Association (NYHA) class improved to class I–II in 96% of patients at one year.
A recent paper, published in JACC: Advances and authored by Nadira Hamid (Minneapolis Heart Institute, Minneapolis, MN), suggests evidence of reverse-remodelling post implantation, Ninios said, with reduction in left atrial volume and left atrial strain, correlating with sustained clinical benefit of improvements in NYHA, Kansas City Cardiomyopathy Questionnaire (KCCQ) scores and six-minute walk tests.
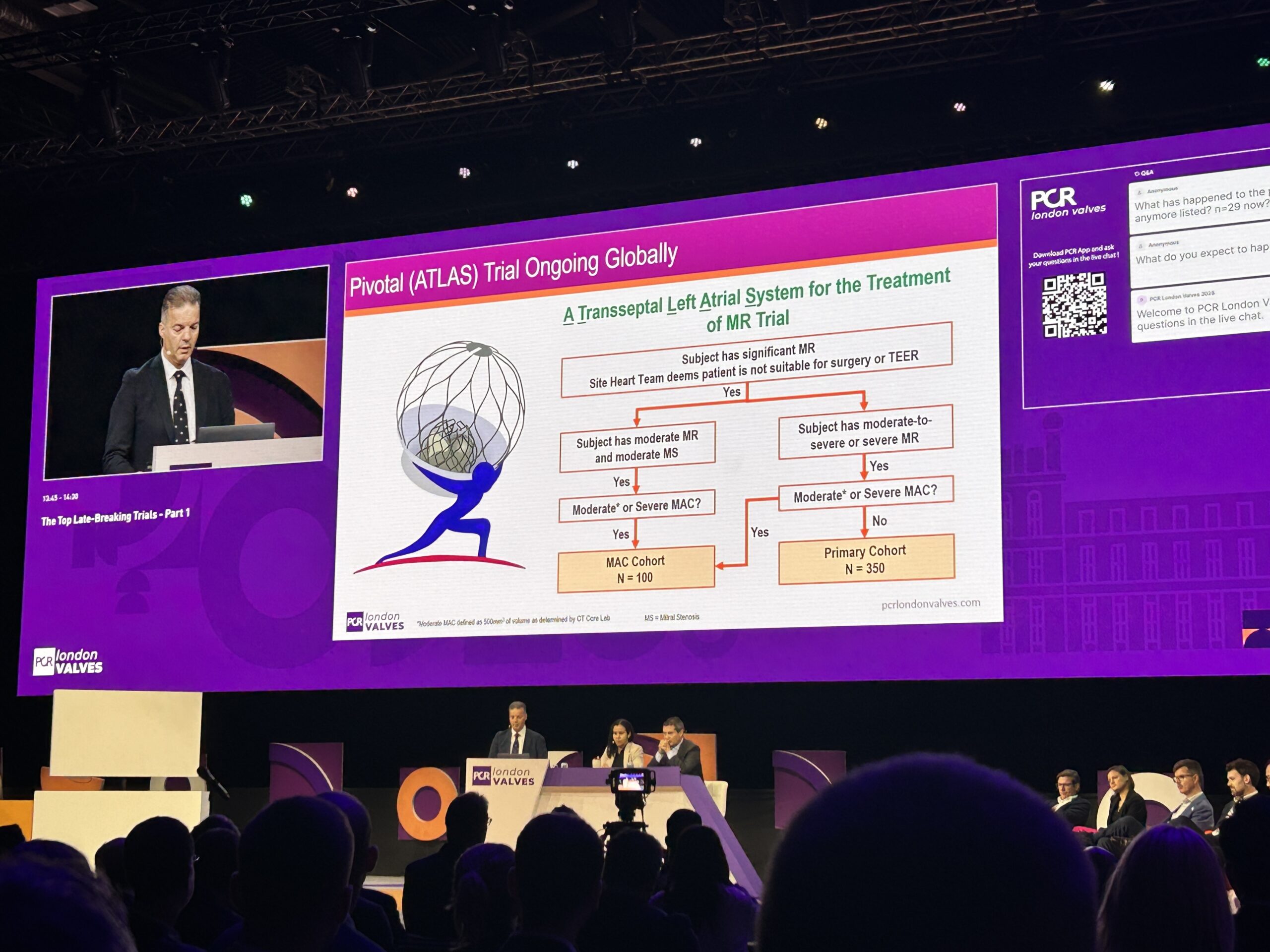
Follow-up from the EFS is expected out to five years, while further study of the device is ongoing with enrolment having begun in the ATLAS pivotal study at European and US sites. ATLAS will include a MAC cohort of 100 patients, as well as a primary cohort of up to 350 patients, Ninios said.
“The one-year outcomes show sustained clinical benefit,” he commented in summary of the overall findings of the study so far. “It’s a heavy burden to do TMVR. We’ve seen that it has been a field that the community has been struggling for years. You really have to carry the weight, and the ATLAS trial hopefully will help us to achieve that.”