This advertorial is sponsored by Medtronic

As transcatheter aortic valve implantation (TAVI) has matured, planning for the lifetime management of patients with aortic stenosis has become a crucial part of the treatment conversation.
“Fifteen or 20 years ago, the surgical community was focused on the haemodynamics of valves,” Danny Dvir (Sha’are Zedek, Jerusalem, Israel) tells Cardiovascular News, looking back over the evolution of treatment options for aortic stenosis, and how the role of TAVI has changed in that time. “With the interventional cardiology community, the focus was stroke, paravalvular leak (PVL) and vascular complications—we never cared about five or 10-year follow-up.”
However, trials including the Evolut Low Risk trial and NOTION, both using versions of Medtronic’s EvolutTM and earlier CoreValveTM family of valves, have demonstrated promising signals over the efficacy of TAVI in patients who have been deemed to be at low risk for surgery and the potential long-term durability of the therapy, contributing to a widening of the scope of TAVI over time to cover a broader population of patients.
“Now that we talk about 65–70-year-old patients, relatively young patients that will outlast their valve performance, we need to think like the surgeons thought 15 years ago,” comments Dvir. “We need to talk about valve performance, we need to talk about durability of valves, we need to talk about reinterventions and what it means to reintervene after the first valve.”
More and more, the interventional cardiology community is grasping the importance of valve durability and haemodynamic performance as they consider the optimal treatment plan for their patients, and multiple studies have shown Evolut, a self-expanding, supra-annular bioprosthetic valve, to offer a favourable performance across these areas.
Two-year results from the SMART trial—the multicentre randomised trial comparing Evolut to the balloon-expandable Sapien (Edwards Lifesciences) family of devices in patients with small aortic annuli—were shared for the first time at the 2025 Cardiovascular Research Technologies (CRT) meeting (8–11 March, Washington DC, USA). The trial enrolled 716 patients with a valve annulus of 430mm2 or less, with a majority female population.
“SMART is a very important study,” says Dvir of the significance of this research, pointing out that patients with small annuli can be more prone to suboptimal haemodynamics after valve replacement in addition to inferior valve durability.
Notably, elevated mean gradient has been associated with reduced survival after surgical or transcatheter valve replacement, whilst severe prosthesis-patient mismatch (PPM) has also been shown to be another factor associated with increased mortality after a TAVI procedure.
SMART’s two-year results appear to validate the haemodynamic performance of Evolut in small annuli patients, demonstrating a rate of only 4.7% haemodynamic structural valve dysfunction (defined as a mean gradient of ≥20mmHg) amongst Evolut patients, compared to 42.4% in the Sapien arm of the trial. Additionally, patients treated with Evolut had an effective orifice area (EOA) of 1.93cm2 on average after two years, compared to 1.51cm2 amongst patients receiving Sapien. Whilst these haemodynamic findings are promising, clinical outcomes remain broadly similar with the two devices, but will continue to be monitored out to the long-term. Interestingly, the rate neurologic adverse events of transient ischemic attacks occurred more commonly in the Sapien group as well as valve thrombosis.
“We need to be very humble,” says Dvir of these outcomes. “When we look at lifetime management, and how haemodynamics matter over the years, two years is really the beginning.
“What is clear in the data is that valve performance differs dramatically between the two devices. It is not a slight difference; it is a dramatic difference between these two devices.”
If SMART offers a reassuring picture of the haemodynamic performance of the Evolut valve, the latest data from the Evolut Low Risk trial, which compared the Evolut device to surgical aortic valve replacement (SAVR) in 1,414 low-risk patients, give an indication of its durability. Presented at the 2025 American College of Cardiology (ACC) scientific sessions (29–31 March, Chicago, USA) and simultaneously published in the Journal of the American College of Cardiology (JACC), the five-year results indicate that patients who were treated with Evolut showed comparable rates of all-cause mortality or disabling stroke at five years (15.5%) to those who underwent surgery (16.4%). Investigators also reported significantly larger EOAs and lower mean gradients in the TAVI arm versus the surgical arm of the trial.
“I think that we should not expect to see major differences in hard endpoints between surgery and TAVI in stroke [or] in mortality,” says Dvir. “Five years is starting to be a meaningful follow-up, [and] we don’t see significant differences between a more invasive approach and a less invasive approach. Why, then, should a patient choose a more invasive approach? There needs to be a very good argument for them to say it is better.”
Coronary access: Evolut FX+ TAVI system
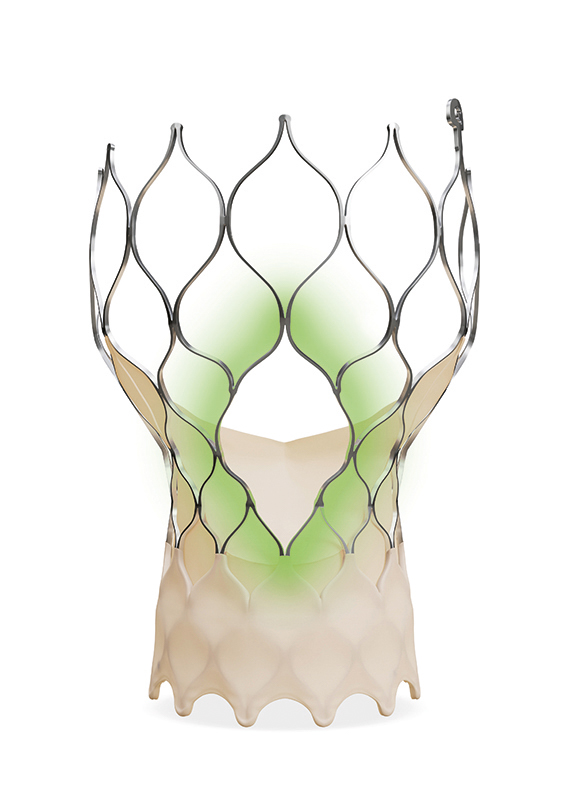
A central facet of any lifetime management strategy is leaving the door open for any future procedures that a patient may require further upstream, for example percutaneous coronary intervention (PCI) in those who may have or develop concomitant coronary artery disease, meaning that the possible need to maintain coronary access is an important consideration as the interventionalist plans their TAVI procedure.
“The ability to do PCI safely is obviously very important. The vessels that supply blood to the heart originate near the aortic valve, and there is a potential for a need for intervening in these vessels,” explains Dvir.
Practice has evolved in this area in recent years with many interventionalists being cognisant of aligning the commissures of the implanted TAVI valve with the native anatomy of the aortic valve to improve future coronary access. Latest iterations of device design are also responding to this need, with the latest-generation Evolut platform—Evolut FX+—offering three larger coronary access windows through a modified diamond-shaped cell design (see figure below), which are four times larger than regular cells of the Evolut TAVI system. This provides increased space for catheter manoeuvrability to facilitate access to coronary arteries of varying patient anatomies, whilst also designed to maintain its structural strength and radial force.
Relaying his experience of these new features, Dvir says: “I can say from personal experience and from communication with colleagues that the issue of PCI after TAVI is becoming less significant than in the past. I personally have done numerous PCIs after Evolut, and quite a few now after FX+, and I don’t see a concern. For me, having previous coronary artery disease and a future need for PCI does not shift the decision about valve selection when I do TAVI, unless there is a unique scenario.”
With physicians increasingly seeking options that give them reassurance over long-term durability and leave the door open for future procedures, latest data and device iterations position Evolut as a prime choice for the lifetime management of patients with aortic stenosis in younger patients.










