 Data indicate that patients who undergo TAVI with the Sentinel Cerebral Protection System (Claret Medical) have a significantly higher rate of stroke-free survival than do patients who undergo TAVI without the device. Furthermore, data also suggest Sentinel captures debris in 99% of patients; for this reason, Samir Kapadia (Cleveland Clinic, Cleveland, USA) believes that the device should be used in all patients.
Data indicate that patients who undergo TAVI with the Sentinel Cerebral Protection System (Claret Medical) have a significantly higher rate of stroke-free survival than do patients who undergo TAVI without the device. Furthermore, data also suggest Sentinel captures debris in 99% of patients; for this reason, Samir Kapadia (Cleveland Clinic, Cleveland, USA) believes that the device should be used in all patients.
The risk of stroke after TAVI is an ongoing concern. While newer generation TAVI devices are associated with a lower risk of stroke than are first-generation devices, the risk of stroke after TAVI is still higher than it is after surgery (in inoperable/high-risk patients). Kapadia observes that this risk is now a “still significant” 3.5% (major stroke 2.5%).
Kapadia and colleagues1 report in the Journal of the American College of Cardiology that stroke “remains a concerning complication”. They add that clinically “silent” brain infarctions—as seen on MRI—occur in “as many as 80% of patients” after TAVI. They observe that these infarctions “are associated with neurocognitive function changes”. “Although the aetiology of strokes and MRI perfusion abnormalities is multifactorial, most are the result of embolisation of debris during the procedure,” the authors add.
The Sentinel device, which is now both CE marked and US FDA cleared, was designed to address the concern of stroke after TAVI; specifically, those strokes related to embolic debris. It consists of two filters within a single 6Fr catheter. This catheter is placed from the right radial or brachial artery over a 0.014-inch guidewire. Prior to TAVI, the filters are positioned in the brachiocephalic and left common carotid arteries; they are then withdrawn into the catheter and removed after the procedure. The safety and efficacy of the device has been demonstrated in several studies.
Evidence base
In CLEAN TAVI,2 100 surgically high-risk patients undergoing TAVI at the University of Leipzig Heart Center, Leipzig, Germany were randomised to receive cerebral protection (50 patients) or no cerebral protection (50 patients). The primary endpoint was the numerical difference in new positive post-procedure diffusion-weighted MRI (DW-MRI) two days after TAVI in protected territories. The investigators, writing in the Journal of the American Medical Association, Stephan Haussig (University of Leipzig, Heart Center, Leipzig, Germany) and others report that the Sentinel device was associated with a significantly fewer new lesions: 4 vs. 10 for the no cerebral protection group (p<0.001). New lesion volume (a secondary endpoint) was also 54% lower: 242mm3 vs. 527mm3 for the control group (p=0.01).
Concluding their findings, Haussig et al observe that the use of the SENTINEL device did reduce the frequency of ischaemic cerebral lesions in protected areas. However, they do state that larger studies “are needed to assess the effect of a cerebral protection device on neurological and cognitive function after TAVI”.2
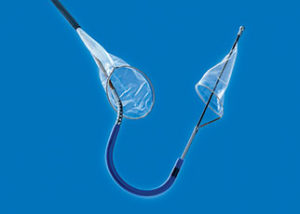
The aim of the Sentinel randomised controlled trial,1 therefore, was to further examine the safety and efficacy of the SENTINEL device in a larger patient population. In this study, patients were randomised into one of three groups: a safety arm (123; all received the Sentinel device), a device imaging arm (121; all received the Sentinel device), and a control imaging arm (119; no patients received the Sentinel device). Unlike the patients in the two imaging arms, the patients in the safety arm did not undergo MRI after the TAVI procedure. Kapadia et al comment that the patients in this arm did not undergo MRI to allow the investigators to “assess safety without increasing the cost of the trial”.
There were no significant differences in the primary safety endpoint—the rate of major adverse cardiac and cerebrovascular events (MACCE) at 30 days—between the device groups and the control imaging group. The primary endpoint was reduction in new lesion volume in protected brain territories (on MRI two to seven days after TAVI) and there were no significant differences between the device-imaging arm and the control-imaging arm in this endpoint.
However, mean new lesion volume was lower in the device-imaging arm compared with the control-imaging arm: 102.8mm3 vs. 178mm3, respectively. This finding meant that the Sentinel device was actually associated with a 42% reduction in new lesion volume. It was also associated with a significant 63% reduction in all-strokes at ≤72 hours (p=0.05).
According to Kapadia et al, several study limitations “likely contributed to the lack of statistical significance” in the reduction of new lesion volume. These include “considerable variance” in MRI post-procedure results, few benchmark MRI data on which to base control arm assumption, and the observed new lesion volume and number were less than predicted from CLEAN TAVI. Kapadia summarises these observations by saying “the primary endpoint was underpowered and we did not have enough prior information; the clinical importance of this endpoint was also unclear”.
In an accompanying editorial3 to the Sentinel study, Azeem Latib (San Raffaele Scientific Institute, Milan, Italy) and Matteo Pagnesi (EMO-GVM, Centro Cuore Columbus, Milan, Italy) agree that an insufficient sample size may have led to the study not meeting its surrogate imaging endpoint. They add that pooling study-level data from three randomised controlled trials reviewing the Sentinel device (SENTINEL, CLEAN-TAVI, and MISTRAL-C) provides a total of 314 patients undergoing TAVI with (165) or without the device (149) and cerebral DW-MRI before and after the procedure. “This meta-analysis suggests that the Sentinel dual-filter device significantly reduces total new lesion volume in protected regions by approximately 100mm3 of damaged brain,” Latib and Pagnesi write.
Furthermore, in the Sentinel study, debris was found in filters in 99% of patients, with Kapadia et al reporting that the debris components included “acute thrombus with tissue elements, artery wall, calcification, valve tissue, and foreign materials”.
They add: “On average, one in four patients had 25 distinct pieces of debris larger than 0.5mm in size. Those are visible to the naked eye and can potentially block mid and distal cerebral arteries downstream.” Also, 16% of the patients had debris larger than 2mm.
Following the publication of the SENTINEL study, Gennaro Giustino (The Zena and Michael A Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, USA) published—in the Journal of American College of Cardiology4—an updated systematic review and aggregate data meta-analysis of five randomised controlled trials (including SENTINEL) of embolic protection during TAVI. Of 625 patients included in the meta-analysis, 376 had been randomised to embolic protection (various devices) and 249 had not. Giustino et al found that embolic protection was associated with a significantly lower risk of stroke or death, corresponding to an approximate 4% absolute reduced risk of stroke (with a number needed to treat of 22). The authors conclude: “The totality of the data suggests that use of embolic protection during TAVI appears to be associated with a significant reduction in death or stroke.”
A real-world study,5 also published after SENTINEL, provided further insights into the safety and efficacy of the device. It showed that using the Sentinel device alongside TAVI is associated with a significantly higher rate of stroke-free survival than is performing TAVI without the device. Overall, the device was associated with a 70% relative risk reduction in stroke/death.
On the basis of the current evidence base, Kapadia told Cardiovascular News that identifying a “high-risk” population that would benefit the most from cerebral protection is difficult. Therefore, he says , “all patients need the device” given that debris was found in filters of nearly all patients—regardless of valve type or STS risk score—who received a device in the SENTINEL study.
References
- Kapadia S, Kodali S, Makkar R, et al. Protection against cerebral embolism during aortic valve replacement. JACC 2017; 69: 367–77.
- Haussig S, Mangner N, Dwyer M, et al. Effect of cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis. JAMA 2016; 316: 592–601.
- Latib A, Pagnesi M. Cerebral embolic protection during transcatheter aortic valve replacement: A disconnect between logic and data? JACC 2017; 69: 78–80.
- Giustino G, Sorrentino S, Mehran R. Cerebral embolic protection during TAVR. JACC 2017; 69: 463–70.
- Seeger J, Gonska B, Otto M, et al. Cerebral embolic protection during transfemoral aortic valve replacement significantly reduces death and stroke compared with unprotected procedures. JACC: Cardiovascular Interventions 2017. Epub.










