 Simpson Interventions has announced that its Acolyte image-guided crossing and re-entry catheter system has been granted breakthrough device designation by the US Food and Drug Administration (FDA).
Simpson Interventions has announced that its Acolyte image-guided crossing and re-entry catheter system has been granted breakthrough device designation by the US Food and Drug Administration (FDA).
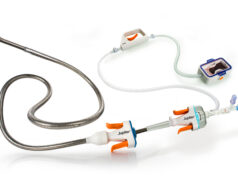
The Acolyte image-guided crossing and re-entry catheter system is designed to facilitate the placement and positioning of guidewires and catheters within the coronary vasculature for the treatment of patients with coronary chronic total occlusions (CTOs) who continue to experience symptoms following medical therapy.
This breakthrough device is intended to significantly change the approach to treating coronary CTOs by providing real-time optical coherence tomography (OCT) visualisation, enabling precise guidewire placement within the target vessel’s true lumen and subsequent revascularisation.
CTOs occur when a coronary artery is completely blocked by plaque buildup, posing significant challenges for interventional cardiologists who struggle to traverse these lesions with current standard of care options.
If a physician fails to cross a CTO, minimally invasive revascularisation options such as angioplasty and stent placement cannot be performed. Hence, in the USA, many CTO patients are instead sent for bypass surgery.
The Acolyte system aims to overcome these challenges by providing clinicians with this breakthrough device that has enhanced visualisation and navigation capabilities, ultimately improving CTO crossing, procedural success rates, and patient outcomes.
“We are thrilled to receive FDA’s breakthrough device designation for our Acolyte image-guided crossing and re-entry catheter system,” said John B Simpson, founder and CEO of Simpson Interventions. “This designation recognises the transformative potential of our technology in addressing a critical unmet need in the treatment of patients with coronary chronic total occlusions. We are committed to advancing the field of interventional cardiology and improving patient outcomes through innovation.”