Use of cerebral embolic protection with the Sentinel (Boston Scientific) device during transcatheter aortic valve implantation (TAVI) did not result in a reduction in the rate of stroke, data from the randomised BHF PROTECT-TAVI trial have shown.
Results from the trial, which is the largest randomised trial to assess the use of a cerebral embolic protection device during TAVI, were presented during a late-breaking trial session at the 2025 American College of Cardiology (ACC) scientific session (29–30 March, Chicago, USA) and simultaneously published in the New England Journal of Medicine.
The trial’s results suggest that there is “No evidence to support a recommendation for the routine use of cerebral embolic protection in TAVI,” investigator Rajesh Kharbanda (John Radcliffe Hospital, Oxford, UK) commented during his presentation.
The results were consistent with those of a prior trial, PROTECTED TAVR, which involved around 3,000 patients from centres in North America, Europe and Australia, had shown that the use of the device did not have a significant effect on the incidence of periprocedural stroke, though a difference in rates of disabling stroke, a secondary endpoint in the trial, was believed to confer some benefit to the use of the technology—at least among advocates of the technology, Kharbanda said.
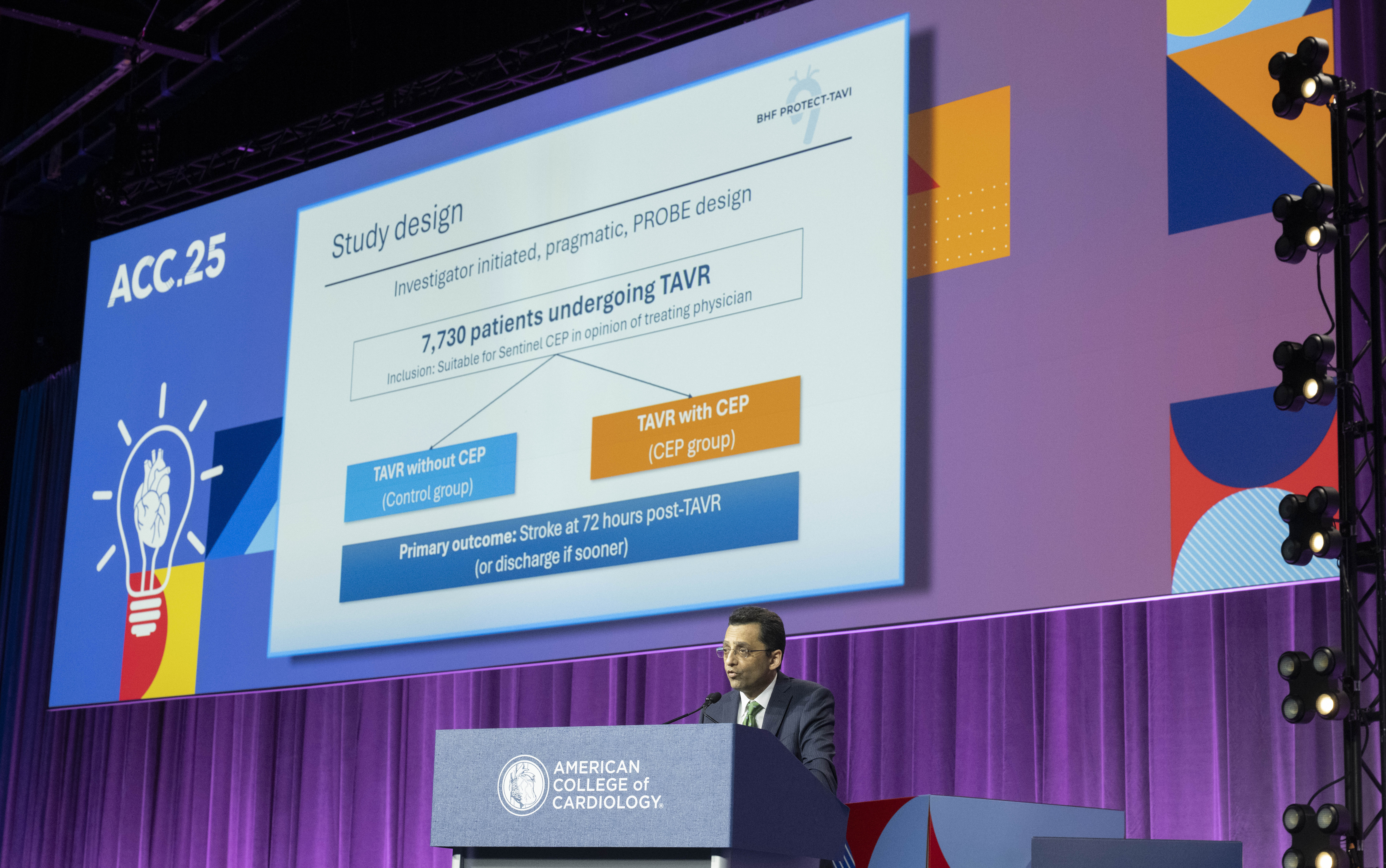
The BHF PROTECT-TAVI trial enrolled 7,635 patients undergoing TAVI for aortic stenosis at 33 centres in the UK between 2020–2024, representing about 30% of all UK patients who received the procedure in those years. Participants had an average age of 81 years and just under 40% were female. Participants were randomised 1:1 to undergo TAVI with the Sentinel device or TAVI without cerebral embolic protection.
In a third planned interim analysis, the trial’s primary endpoint—the incidence of stroke at 72 hours post-TAVI or at the time of hospital discharge, if sooner—occurred in 2.1% of patients assigned to the Sentinel device and in 2.2% of patients assigned to the control group, showing no evidence of a difference between the two study arms. Results for secondary endpoints including all-cause mortality, stroke severity, disabling stroke and cognitive outcomes were also similar between groups. Recruitment for the trial was stopped after the interim analysis based on these findings.
No differences in outcomes were seen in any subgroups that were analysed.