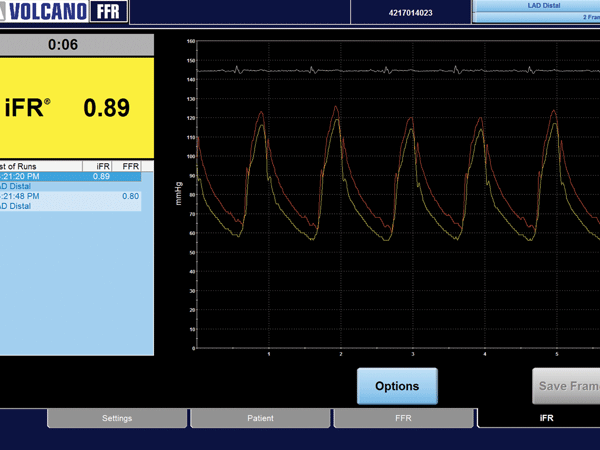
 Economic data from the DEFINE FLAIR clinical trial indicate that using instantaneous wave-free ratio (iFR) provides one-year average cost savings of US$896 per patient compared with a strategy of using fractional flow reserve (FFR). The data were presented at the 2018 American College of Cardiology (ACC) Scientific Session (10–12 March, Orlando, USA).
Economic data from the DEFINE FLAIR clinical trial indicate that using instantaneous wave-free ratio (iFR) provides one-year average cost savings of US$896 per patient compared with a strategy of using fractional flow reserve (FFR). The data were presented at the 2018 American College of Cardiology (ACC) Scientific Session (10–12 March, Orlando, USA).
DEFINE FLAIR is a randomised, controlled, single-blinded comparison of clinical outcomes and cost efficiencies of iFR and FFR-guided interventions of 2,492 patients in 49 centres across Europe, Asia, North America, and Africa. The study conducted its comparison of iFR vs. FFR using pressure guide wires and equipment from Philips.
With an average saving of nearly US$900 per patient per year, the study found that iFR offers a total procedure cost saving of approximately 10% per patient over FFR. Additionally, patients treated with the use of an iFR-guided revascularisation strategy had fewer coronary artery bypass graft procedures and fewer subsequent revascularisations. Previous data from DEFINE FLAIR released in 2017 found that iFR-guided treatments reduced procedure time by 10% vs. FFR-guided treatments, while reducing patient discomfort by 90%. The pivotal DEFINE FLAIR study continues to illustrate the advantages of iFR and the superior value it delivers to clinicians and hospital administrators.
Manesh Patel (Duke Heart Center, Duke University School of Medicine, Durham, USA), says: “The findings from DEFINE FLAIR continue to demonstrate the benefits of iFR—showing that an iFR-guided treatment offers proven outcomes, reduced costs and procedure time, and enhanced patient comfort compared to FFR. iFR is not only a faster diagnostic solution, but it also offers the advantage of significantly reduced patient discomfort. By implementing an iFR programme at a hospital, this solution can deliver the clinical outcome benefits of physiology-driven PCI, while reducing annual health care costs significantly across the organisation.”
Since the introduction of the hyperaemia-free iFR modality in 2013, a press release reports, iFR has been studied in nearly 15,000 patients and is used in more than 4,100 catheterisation labs across the world.
“The DEFINE FLAIR study has provided further clinical validation of iFR and how it is improving the lives of patients and physicians. An iFR-guided strategy has now been shown to improve patient outcomes and reduce costs for the treatment of coronary artery disease in comparison to an FFR-guided strategy. This is a significant step in our journey to help clinicians decide, guide, treat and confirm the right therapies for their patients, while reducing costs,” comments Christopher Barys, Business Leader of Philips Image Guided Therapy Devices.
iFR was developed by Volcano, who were bought by Philips.