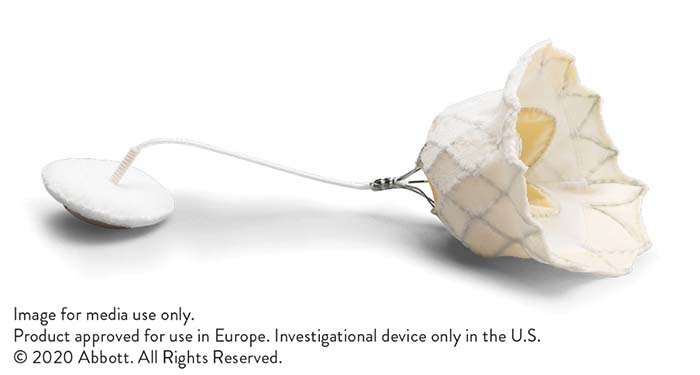
 The Tendyne transcatheter mitral valve implantation (TMVI) system (Abbott) has received the CE mark, making Abbott the first company to have such a device on a market anywhere in the world. A press release reports that this “life-changing” therapy treats significant mitral regurgitation in patients requiring a heart valve replacement and provides a safe and effective solution for patients who are not candidates for open-heart surgery or transcatheter mitral valve repair.
The Tendyne transcatheter mitral valve implantation (TMVI) system (Abbott) has received the CE mark, making Abbott the first company to have such a device on a market anywhere in the world. A press release reports that this “life-changing” therapy treats significant mitral regurgitation in patients requiring a heart valve replacement and provides a safe and effective solution for patients who are not candidates for open-heart surgery or transcatheter mitral valve repair.
For patients at high-risk for open-heart surgery or in clinical situations when the mitral valve is too damaged for a successful repair with Abbott’s MitraClip device, according to a press release, the Tendyne system offers a much-needed, alternative minimally invasive treatment option when the leaky valve needs to be replaced.
The Tendyne valve is a first-of-its-kind therapy to replace the mitral valve in patients in need of symptom relief and quality-of-life improvement without open surgery and when transcatheter mitral repair is not possible. Global trial results to date have demonstrated excellent procedural safety and have shown 98.9% of Tendyne patients experienced mitral regurgitation elimination at discharge which was sustained through one-year in this very sick patient group.
The system is designed to adapt to a range of patient anatomies, the press release says, thanks to its innovative and unique design. The self-expanding valve is delivered through a small incision in the chest and up through the heart where it is implanted in a beating heart, replacing the person’s native mitral valve. It is available in multiple sizes to treat a broad range of valve anatomies. The valve is fully repositionable and retrievable during implantation, allowing for the best possible outcome for people suffering from mitral regurgitation and needing a valve replacement.
Hendrik Treede (University Hospital Bonn, Bonn, Germany) says: “European approval for Abbott’s Tendyne mitral valve replacement therapy provides the clinical community with a new choice in how we approach correcting a leaking mitral valve. For the first time outside of clinical trial settings, heart teams now have a minimally invasive valve replacement therapy that is backed by an excellent safety profile and designed to help physicians reposition the device as needed for improved patient outcomes.” A post-approval study of the therapy will also be co-led by Nicolas Dumonteil (Clinique Pasteur, France).
Michael Dale, senior vice president of Abbott’s structural heart business, comments: “The launch of the Tendyne device builds upon our history of developing ground-breaking therapies that offer new treatment options for people with serious structural heart conditions who have limited treatment options. The availability of Tendyne as a treatment option in Europe provides physicians with an additional tool that has been shown to completely correct mitral regurgitation in very ill patients, and it adds to Abbott’s portfolio of life-changing and life-saving treatments.”











