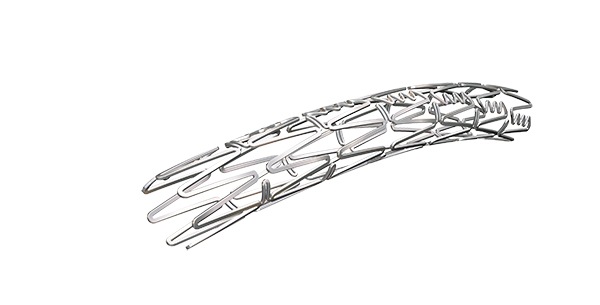
A 24-day old infant has received the first-in-human placement of BeGrow (Bentley), a novel stent system that grows with the child, to alleviate pulmonary artery stenosis.
According to a company release, BeGrow is small enough to fit a newborn’s 6mm diameter pulmonary artery, yet can be post-dilated as the child grows up to a vessel diameter of 11.5mm (around 8–10 years of age). Beyond this diameter, the stent breaks open at pre-determined points in a controlled way. This feature makes the stent unique, and particularly suitable for when young patients transition to adults without having to experience open surgery and the associated risks.
The first-in-human procedure marks the start of the BeGrow clinical trial performed by principal investigator and interventional paediatric cardiologist, Professor Oliver Kretschmar from the Kinderspital Zurich, Switzerland.
“We have been looking for something to fulfil the need for a stent that can grow with the vessel from infant to child to adult,” says Professor Kretschmar. “With BeGrow, the stent ‘breaks open in a controlled way’ when it has been dilated to a diameter >11.5mm, after which point it is designed to grow with the child’s vessel, as pig studies have shown. If needed, we could place an adult-width stent in the pulmonary artery, while BeGrow remains in situ,” he continues, noting that the clinical trial results would determine the preferred course of action in the long-term.
“The benefit of the BeGrow is that the stent can stay in the patient for the rest of their life,” he adds. “Even if we add-in another stent of adult size, it is certain that we will not have to surgically remove the BeGrow, like we do with other current off-label solutions, inherently carrying a risk of vessel damage,” he says.
The BeGrow stent system should be indicated for intraluminal placement in the pulmonary arteries of newborns and infants for the treatment of pulmonary artery stenosis, an indication that meets an unmet medical need. BeGrow is balloon expandable with a low crimped profile that can be inserted and dilated at minimal pressure via the 4 French (F) compatible catheter to 6mm expanded diameter, the width of a newborn pulmonary artery. The stent is available in lengths of 10, 13, 17, 20, and 24mm.
BeGrow is aimed at treating babies with congenital heart disorders, which has a prevalence of approximately one in 100 newborns in Europe. Few medical device companies have developed devices for paediatric cardiology because the market is so small.
Christian Bader is BeGrow product manager and former research and development project leader for Bentley. “Due to the extremely small diameter of an infant’s blood vessels, there is currently only one option for these patients, which is a coronary stent,” he explains. “However, these stents can’t grow to an adult vessel diameter, hence surgical removal is required. At Bentley we have focused our research and development on finding a solution to meet this need for paediatric cardiologists to improve the clinical outcome and quality of life for the patient.”
BeGrow clinical study
Research into BeGrow began in 2012, and was conceptualised and designed by Bentley’s co-founder, Miko Obradovic. Technical development was complete in 2017 and the clinical trial began that year at the Kinderspital Zurich, with two sites in Germany and one in Austria to be added soon.
The BeGrow clinical study is a prospective, multicentre, explorative, open label single-arm study to assess the safety and performance of the BeGrow Stent System for newborns and infants in pulmonary artery stenosis. The primary outcome measures are vessel enlargement directly after procedure, and during follow up to 12 months. A total of 18 infants will be included.
“Bentley will submit for CE immediately after the 12-month results [primary outcome measures], and will continue to gather long term results of efficacy and safety up to 8–10 years of age,” says Natasa Mitrovic, clinical affairs manager for BeGrow.
“Currently, standard of care in infants comprises stents neither made nor indicated for infants. They are only designed for adults,” reflects Professor Kretschmar. “With children, especially the very young, we have to customise adult products but the diameter of these stents is restricted, and with over-dilation we lose function. We then need to add-in an adult stent, but because the size of the original stent is restricted, the vessel can remain narrowed.” The continued growth of BeGrow within the vessel wall, after 11.5 mm, is designed to overcome these issues. A further advantage of BeGrow, Professor Kretschmar adds, is the possibility of using a very small 4F vessel sheath to enter the vessel as opposed to a standard large sheath that can damage a vessel upon insertion. “The result in the first case was excellent,” he reports. “We hope that this development means we can implant a stent at a very early age and this can grow with, and stay with, the patient for the rest of their life, but of course, long-term results need to be shown in trials.”











