Early experiences with two transcatheter mitral valve implantation (TMVI) devices—Intrepid (Medtronic) and the Tendyne mitral valve (Abbott)—indicate that TMVI may be a safe and effective approach for managing mitral regurgitation in patients at high risk for surgical complications. However, further studies are needed to support these findings.
Presenting the results of the Intrepid Global Pilot Study at the 2017 Transcatheter Cardiovascular Therapeutics (TCT) meeting (29 October–2 November, Denver, USA), Paul Sorajja (Minneapolis Heart Institute Foundation, Abbott Northwestern Hospital, Minneapolis, USA) reported that TMVI had “recently emerged” has a potential therapy for mitral regurgitation but noted that the feasibility of the approach “remains uncertain”.
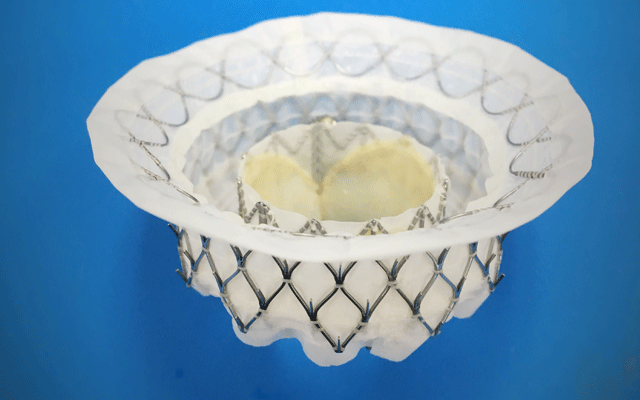
The aim of the Intrepid Global Pilot Study, therefore, was to explore the feasibility of Medtronic’s Intrepid device. Sorajja noted that the device had a self-expanding nitinol frame (43, 46, or 50mm diameter), housed a 27mm trileaflet bovine, and was delivered via a transapical system using a 35Fr catheter. He added that in the study, 50 patients with severe mitral regurgitation and who were at high or extreme risk for surgery underwent TMVI with device.
There were seven deaths within 30 days (one of which was due to device malaposition) and 11 deaths after the 30-day timepoint. However, there were no further deaths after the first 81 days—meaning that the rate of one-year survival was 76.5%. According to Sorajja, the device was also associated with a significant improvement in mitral regurgitation severity. At the last follow-up point, 26.2% of patients had mild regurgitation and 73.8% had no regurgitation vs. 4.1% with moderate regurgitation and 95.9% with severe regurgitation at baseline. Furthermore, there was a significant improvement in New York Heart Association class with 79% of patients in Class I or II at follow-up.
Sorajja commented: “TMVI with the Intrepid valve was feasible and resulted in correction of mitral regurgitation in symptomatic patients at high or extreme surgical risk. Stable valve function was observed and a majority of patients experienced symptoms improvement.” He added that further investigations “will determine the role of this therapy in a broader patient population compared with surgery and other transcatheter techniques”.
Prior to the presentation of the study, which was simultaneously published in the Journal of the American College of Cardiology, Medtronic announced that the first patient had been enrolled in the APOLLO trial of the Intrepid device. The study, a press release reports, will have two cohorts: a randomised cohort that will compare TMVI with Intrepid to mitral valve surgery in surgical candidates who are not eligible for repair; and an observational cohort of patients who are inoperable (as determined by the heart team) undergoing TMVI with the device. David H Adams (Mount Sinai Health System, New York , USA), national co-principal investigator of the trial, comments: “This is the beginning of an important journey to establish a truly less invasive approach to treat severe mitral valve regurgitation in patients who are appropriate candidates for mitral valve replacement with a transcatheter technology that eliminates the need for open-heart surgery.”
Tendyne
Data for the Tendyne TMVI device were also presented at the TCT meeting. David Muller (St Vincent’s Hospital, Sydney, Australia) reported that the device—similar to the Intrepid device—had a self-expanding frame and a porcine pericardium valve. He noted that its advantages were that there was no need for a heart-lung bypass, it enabled treatment of surgically ineligible patients, and it was fully retrievable and repositionable.
Overall, 30 patients received the device but implantation was not successful in two patients (6.7%). By one year, five patients had died (16.7%) and three had been rehospitalised for heart failure (10%) but none for mitral valve surgery. At one year, of the 22 patients for whom data were available, 96% had no mitral regurgitation (compared with none at baseline) and 95% were in NYHA Class I or II (vs. 0% at baseline). Improvements in heart function, heart size, and quality of life were also observed.
Regarding both studies, Sorajja told Cardiovascular News: “The feasibility of these therapies, which are incredibly novel, has been demonstrated, and there is the potential to impact millions of lives, should the upcoming clinical trials demonstrate efficacy and safety.”