In an open public hearing of the FDA’s circulatory system devices panel, according to a press release, nearly all panel members recommended the de novo application of the Sentinel cerebral protection system (Claret Medical) be granted. If the application is granted, the system could become commercially available in the USA. However, a formal vote was not taken as the Sentinel system is a medium-risk accessory device reviewed as a de novo application.
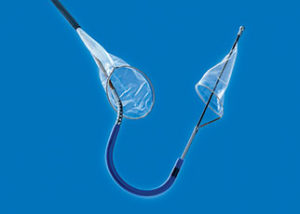
Sentinel is designed to protect the brain by capturing and removing debris dislodged during transcatheter aortic valve implantation (TAVI) that would otherwise enter the cerebral circulation and increase the potential for stroke. In the SENTINEL trial, the primary safety endpoint of major adverse cardiac and cerebrovascular event (MACCE) at 30 days was met, with reported MACCE in the protected Sentinel group at 7.3%, significantly lower than the prespecified historical performance goal of 18.3% and the rate of 9.9% seen in the control arm. The stroke rate for Sentinel-protected patients was 5.6% vs. 9.1% for unprotected patients. Importantly, the observed periprocedural stroke rate—encompassing 72 hours’ post-procedure—was reduced by 63%, from 8.2% for unprotected patients to 3% for protected patients.
In the trial, device delivery and retrieval were accomplished safely and successfully in 99.6% of cases. The Sentinel access site complication rate was only 0.4%. The device captured and removed embolic debris in 99% of patients in the study, with one in four patients having more than 25 pieces of debris with a size greater than, or equal to, 0.5mm captured in the filters.
Martin Leon (Columbia University Medical Center/New York-Presbyterian Hospital, New York, USA), says: “The Sentinel device has shown its potential, across multiple trials, to filter and remove brain-borne debris safely, with very few vascular complications. When we see the size and heterogeneity of the material captured, it is reassuring for me as a practicing TAVI implanter to know that it can be removed from the patient’s vasculature before it reaches the brain.”










