 PlaqueTec has announced new research findings from interim data analysis of its ongoing BIOPATTERN trial, which is aiming to improve understanding of the pathobiology of atherosclerotic cardiovascular diseases.
PlaqueTec has announced new research findings from interim data analysis of its ongoing BIOPATTERN trial, which is aiming to improve understanding of the pathobiology of atherosclerotic cardiovascular diseases.
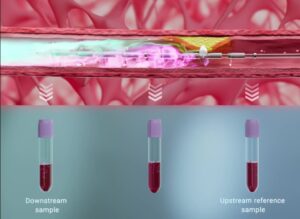
Early insights into the data analysis, following the collection of peri-plaque blood samples from 33 of the first 34 patients enrolled in the trial, has revealed distinct protein gradients in diseased coronary artery blood compared with systemic blood.
The analysis also revealed differences in protein concentration between samples taken simultaneously upstream and downstream of coronary plaque, providing early evidence that this approach can detect proteins concentrating at sites of coronary plaque, PlaqueTec has detailed in a press release. Collection of the first patient samples also confirms the safety profile of PlaqueTec’s liquid biopsy system, used to collect the samples, supporting continuation of the trial, the company states.
The BIOPATTERN trial is designed to investigate trans-plaque protein signals in coronary blood samples from patients undergoing a percutaneous coronary intervention (PCI) procedure. Using the data analysed from the thousands of proteins and other blood molecules measured in each sample, PlaqueTec aims to generate novel site-of-disease insights to improve understanding of mechanisms involved in coronary artery disease (CAD) progression towards heart attack.
The findings will be integrated into a new data platform, which will be used to inform patient stratification and therapeutic development programmes to enable a precision medicine approach to CAD.
These latest findings were presented at the European Atherosclerosis Society congress (4–7 May, Glasgow, UK).
“The current approach to treating CAD involves standardised risk-factor management, leaving many patients vulnerable and at risk of progression towards heart attack. Our approach aims to establish disease-specific treatments to address the residual risk and improve outcomes for patients with CAD,” comments Diane Proudfoot, chief scientific officer, PlaqueTec. “Continuation of the BIOPATTERN trial will allow us to generate a more detailed picture of the pathobiology of CAD, by uniquely sampling blood from the disease microenvironment, to identify distinct disease endotypes and enable the development of precision medicines.”
“The interim analysis marks a key milestone in our ongoing BIOPATTERN trial,” adds Simon Williams, general manager, PlaqueTec. “The safety profile of the liquid biopsy system and high-quality of samples collected so far, and the novel site-of-disease insights generated, provides us with confidence in our approach as we progress towards the 300-patient target.”










