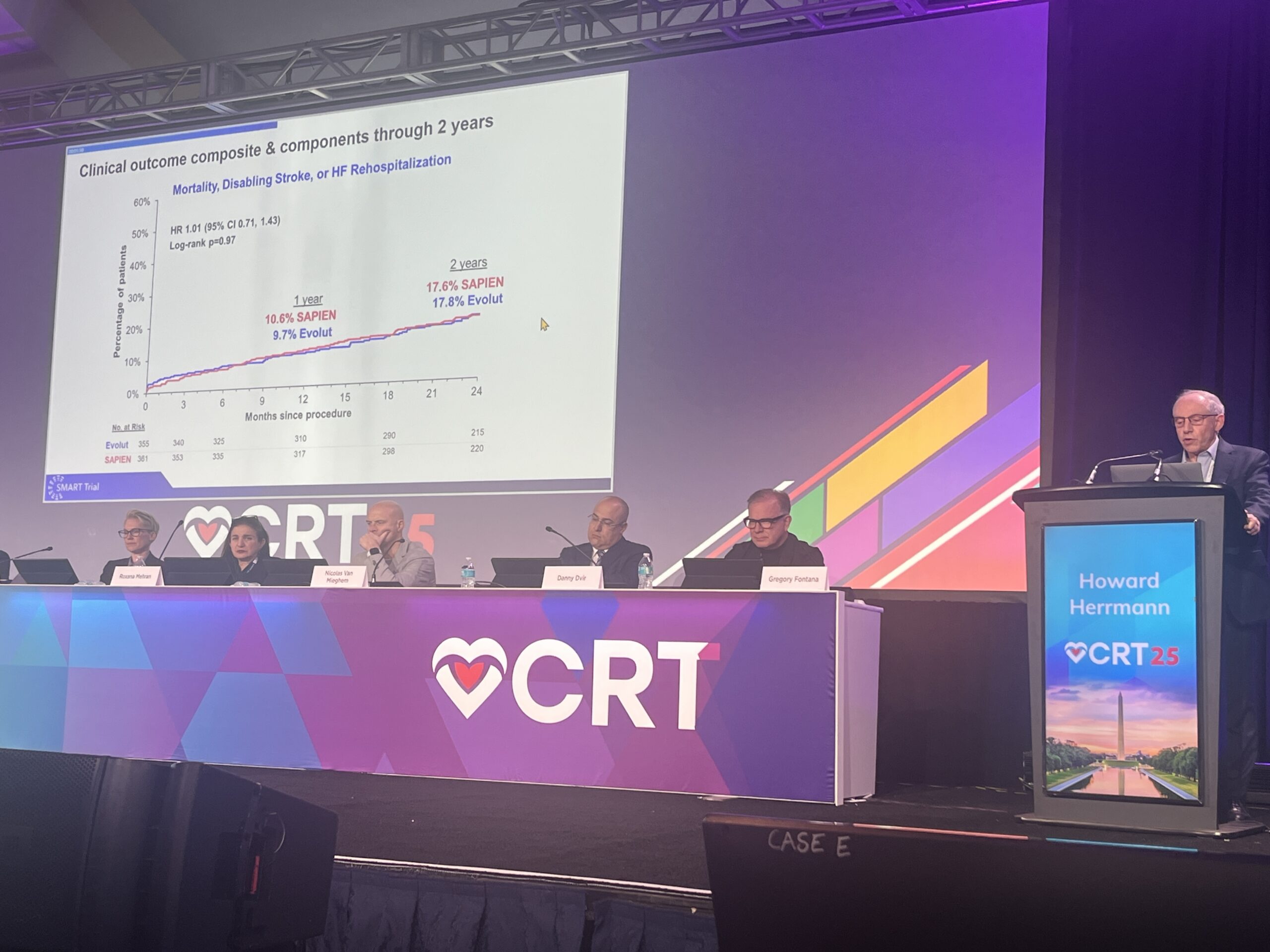
Two-year results of the SMART trial, comparing two commonly used transcatheter aortic valve implantation (TAVI) systems for the treatment of aortic stenosis in patients with small aortic annuli, have shown that though the use of a supra-annular valve demonstrated an improvement on haemodynamic performance measures compared to a self-expanding platform, rates of mortality, disabling stroke and heart failure hospitalisation remain comparable between the two systems.
The SMART randomised trial compared the supra-annular self-expanding Evolut (Medtronic) family of TAVI valves, which includes the Evolut PRO, PRO+ and FX iterations of the system, and the balloon-expandable Sapien 3 and Sapien 3 Ultra (Edwards Lifesciences). The trial is the first to focus specifically on informing device selection for patients with small aortic annuli, a patient group that is primarily women and has been underrepresented in previous clinical trials for TAVI.
Howard Herrmann (Perelman School of Medicine at the University of Pennsylvania, Philadelphia, USA), the trial’s lead investigator, presented the trial’s two-year results at Cardiovascular Research Technologies (CRT) 2025 (8–11 March, Washington DC, USA), where he commented that he did see the potential for the superior haemodynamic performance of the device, which was determined by measuring mean gradient and effective orifice areas, would lead to a benefit in outcomes over time, albeit likely to be more evident later on in the trial’s five-year lifespan.
“Valve performance is critical for all patients, but the impact of poor valve performance is magnified in patients with a small aortic annulus, who are at risk of receiving a valve that is not adequate for their cardiac requirements,” Herrmann said. “The two-year results highlight the continued superior performance of the Evolut TAVR valve in these patients. While we would not yet expect to see a significant difference in the composite clinical outcomes at this early stage, valve performance provides important data that operators can use to inform and personalize treatment decisions to enhance patient outcomes.”
The SMART two-year data demonstrated that Evolut shows less bioprosthetic valve dysfunction, which can be a predictor of adverse outcomes, compared to Sapien. Additional results demonstrated that Evolut showed five times less prosthetic valve thrombosis (p=0.0048), and nine times less haemodynamic structural valve dysfunction (defined by mean gradient ≥20mmHg; p<0.001).
“The SMART Trial is a groundbreaking, head-to-head comparison that, for the first time, highlights how a patient with a small annulus can benefit from Evolut’s differentiated valve design,” said Kendra J Grubb, vice president and chief medical officer, Structural Heart, which is part of the Cardiovascular Portfolio at Medtronic. “At two years, we continue to see superior valve performance that we will follow to assess long-term outcomes.”













