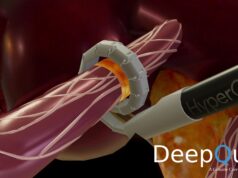
New research will investigate the safety and efficacy of multi-organ, hepatic artery and renal artery denervation using the Symplicty Spyral (Medtronic) catheter in uncontrolled hypertension patients who are both on and off medications.
A planned global pilot study, SPYRAL GEMINI, represents the latest frontier in sympathetic denervation research, following the US Food and Drug Administration (FDA) approval of the Symplicity Spyral and Paradise (Recor Medical) renal denervation systems in late 2023. SPYRAL GEMINI will involve only the use of the Symplicity Spyral catheter.
“It is a nice way of saying that the story doesn’t stop here with renal denervation. We are exploring other vascular beds for renal denervation therapy, and it so happens that the hepatic artery is highly innervated with sympathetic nerves and would provide a rich target for sympathetic denervation,” David Kandzari (Piedmont Heart Institute, Atlanta, USA), who has investigated renal denervation using the Medtronic system, tells Cardiovascular News.
“The pre-clinical data would suggest that hepatic denervation combined with renal denervation may not only achieve a potentially greater reduction in blood pressure, but an even more consistent reduction in blood pressure as well.”
The study programme is anticipated to begin in late 2024/early 2025 and will be “exciting with regard to seeing what the reductions in blood pressure are beyond renal denervation only”, according to Kandzari, and whether this may increase the number of individuals with a response from the therapy.
Kandzari recently presented two-year data from the SPYRAL HTN-ON MED clinical trial at TCT 2024 (27–30 October, Washington, DC, USA). The trial investigated the safety and efficacy of renal denervation using the Symplicity Spyral radiofrequency catheter in 337 patients with uncontrolled hypertension prescribed one to three hypertensive drugs, who were randomised 2:1 to either renal denervation or a sham procedure.
The findings showed that, compared to patients treated with a sham procedure, those who underwent renal denervation had significantly greater reductions in 24-hour ambulatory systolic blood pressure and office-based blood pressure at two years.
The data showed significant group differences in 24-hour ambulatory systolic blood pressure and office-based systolic blood pressure in favour of renal denervation, despite significantly more medications detected in the sham group.
Ambulatory systolic blood pressure reduced by -12.1mmHg in the renal denervation group vs. -7mmHg in the sham group (treatment difference: -5.7 mmHg; p=0.039), and office-based systolic blood pressure: -17.4mmHg in the renal denervation group vs. -9mmHg in the sham group (treatment difference: -8.7mmHg; p=0.0034). Long-term safety was very favourable with no confirmed renal artery stenosis greater than 70% in the Spyral group at two years.
“Now that renal denervation has been approved with FDA regulation and made commercially available in the USA, broadening its use in more clinical applications worldwide, for many practitioners there remain outstanding issues with regard to the long-term durability of this therapy,” says Kandzari of the significance of the study’s findings. “Equally important is the long-term safety as well as other issues such as identifying which patients might benefit most from the therapy.”
“At least in this regard, the two-year outcomes for the SPYRAL ON-MED expansion study, representing the largest contemporary comparative on-med clinical trial, highlights the sustainability of blood pressure lowering, if not [the] amplified blood pressure reductions over a long-term follow-up, which is quite a consistent finding during follow-up in the SPYRAL programme.”
This is consistent with findings from other clinical trials, Kandzari says, as well as from the nearly 3,000-patient Global SYMPLICITY registry. The two-year SPYRAL ON-MED data also confirm the safety of renal denervation, he adds, showing that there are no late-term consequences of the therapy regarding renal artery stenosis or declining kidney function.
“If anything, we would hope that kidney function might be more preserved compared with those individuals with uncontrolled hypertension that poses a continued risk for them. And then ideally too, spending a greater time with blood pressure control or what we call time in target range, or time in therapeutic range, that this may translate to improved clinical outcome for patients.”
With regard to the uptake of the therapy, Kandzari says that, in the USA at least, reimbursement remains the biggest sticking point. This situation shows signs of shifting, with the Centers for Medicare and Medicaid Services (CMS) having this month (November 2024) granted transitional pass-through (TPT) payment for both Medtronic’s Symplicity Spyral and Recor Medical’s Paradise catheters. TPT payment, which will be effective for up to three years beginning 1 January 2025, aims to support patient access to new and innovative technology.
“Receiving TPT approval for our renal denervation catheter is an important milestone for the Symplicity blood pressure procedure, as it will enable greater patient access to a breakthrough treatment by reducing cost barriers for healthcare systems,” said Jason Weidman, senior vice president and president of the coronary and renal denervation business within the cardiovascular portfolio at Medtronic, in a company press release. CMS has created a distinct category and code for ultrasound renal denervation in recognition of the differentiated technology and procedure with Paradise.
“TPT for ultrasound renal denervation increases access to a proven device-based hypertension treatment option to patients who have been unable to achieve blood pressure control with lifestyle changes and medications alone,” said Lara Barghout, president and chief executive officer of Recor Medical.
“The granting of TPT highlights the safety and efficacy of this breakthrough device, which together demonstrated that the Paradise ultrasound renal denervation system met the newness and significant clinical improvement criteria. By creating a distinct device category, CMS have also recognised that the Paradise ultrasound renal denervation system is a highly differentiated technology and that there are significant differences in comparison to other technologies available in the marketplace. This is a major step forward in the reimbursement available for the Paradise ultrasound renal denervation system, creating additional financial support for hospitals and physicians to provide this novel and effective therapy to their uncontrolled hypertension patients.”