This advertorial is sponsored by Medtronic

As transcatheter aortic valve implantation (TAVI) continues its evolution interventionalists need the latest generation of devices to offer features that respond to the needs of a diverse and changing population of patients. The EvolutTM (Medtronic) family of TAVI devices is among the most widely used TAVI systems in the world, and new iterations of the platform have sought to match the developing needs of users whilst maintaining the durability and reliability that physicians and patients expect from previous generations.
Interventional cardiologist Angela McInerney (Galway University Hospital, Galway, Ireland) spoke to Cardiovascular News about the features she sees as being important in the Evolut system and how newer iterations of the device potentially broaden the scope of the patients who may be offered treatment.
Evolut FX was launched in late 2023, bringing with it a series of features intended to enhance ease-of-use and control for operators. “In terms of redesigned features, Evolut FX includes a longer nose cone and a single spine delivery shaft, which both improve flexibility,” McInerney says. These enhancements help to increase the crossability of the device improving its ability to navigate tortuous and calcified anatomies or horizontal aortas, for example.
“The flexibility of the device gives us confidence to use it in these commonly encountered tortuous anatomies, knowing that the device will safely navigate the iliofemoral and aortic angles without the need for a large sheath,” McInerney comments. “This is a major change in comparison to previous Evolut iterations.”
A further addition in Evolut FX is that of radiopaque markers—gold markers located close to the inflow edge of the valve and under the commissures that are visible on fluoroscopy to help operators align the valve with the native annular plane—with the intention of achieving commissural alignment to improve future access to the coronary arteries.
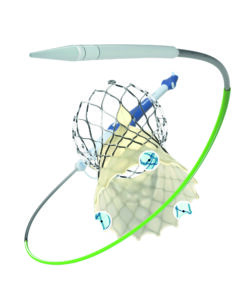
“Commissural alignment has become so much more important for TAVI implanters, particularly when you are thinking about patients who have concomitant coronary artery disease, but also younger patients who could easily come back in the future with myocardial infarction (MI) or angina, where you not only want to image the coronary arteries, but also treat them,” says McInerney, commenting that the addition of radiopaque markers takes any concern off the table about future coronary access.
“I find achieving commissural alignment to be much less challenging and this has resulted in a change in our practice whereby we now perform percutaneous coronary intervention (PCI) in those with concomitant coronary artery disease (CAD) after the TAVI has been implanted,” she explains, adding that the likely ability to achieve commissural alignment also has a bearing on valve choice when considering treatment strategies in younger patients.
The Galway team has been using the Evolut FX platform since its launch over one year ago and McInerney says that she has found the deliverability of the device to be a positive change. The ability to navigate tortuous and calcified anatomies has also been impactful on her practice given the proportion of patients that come into the cath lab with comorbidities that may predispose them to heavily calcified vasculature, such as advanced age, diabetes or chronic kidney disease.
Whether these new features translate into improved patient outcomes remains to be borne out in practice, but McInerney comments that being able to treat more patients with the self-expanding Evolut platform will likely be a plus given that the new device builds upon the legacy Evolut and CoreValve technologies, underpinned by a solid body of data. “The clinical data for the use of Evolut is continuing to grow and be reassuring,” she says. “Many of the recent studies have answered research questions that are very important for TAVI operators.”
Recent data releases support the performance of the Evolut family of devices across a broad range of patient groups, in particular low-risk patients and those with small aortic annuli, as well as highlighting the durability of the prosthesis when compared to surgical aortic valve replacement (SAVR).
Four-year findings from the EVOLUT Low Risk trial—a randomised, non-inferiority trial, comparing TAVI (CoreValveTM, Evolut R, or Evolut PRO) devices to SAVR in low-risk patients with severe aortic stenosis—shared in late 2023, have shown that TAVI patients had a 26% reduction in hazard for death or disabling stroke compared to surgery, with the difference between the two groups continuing to increase over time.
Data on durability and valve performance also appear favourable. A five-year pooled analysis taken from the CoreValve US High Risk and SURTAVI randomised trials has demonstrated a significantly lower cumulative rate of bioprosthetic valve deterioration compared to surgery, as well as five-year rates of structural valve deterioration and non-structural valve dysfunction that were significantly lower after TAVI compared to surgery.
McInerney says that the evidence of a divergence of the curves for all-cause mortality and stroke seen in the EVOLUT Low Risk trial can be considered “good news” for patients, whilst the haemodynamic results seen with the device also provide reassurance.
More recently the interventional community has digested findings from the SMART trial, the randomised trial in which Evolut PRO, PRO+ and FX systems were compared against the balloon-expandable Sapien 3 and Sapien 3 Ultra (Edwards Lifesciences) devices in patients with small aortic annuli. Though demonstrating a similar rate of death, disabling stroke or rehospitalisation for heart failure in both groups, Evolut was shown to be superior in bioprosthetic valve dysfunction through 12 months, with a rate of 9.4% compared to 41.6% with the balloon-expandable valve.
“Comparison between TAVI devices is always of interest to us as TAVI operators. We want to be confident in our choice of valve to ensure the safety of our patients as well as providing long-term durability,” says McInerney of the results. Patients with small annuli—a group that is disproportionately made up of women—often presents a challenge for TAVI operators, where optimising haemodynamic performance and avoiding patient prosthesis mismatch (PPM) is important. Crucially, SMART demonstrates a positive impact for Evolut in both.
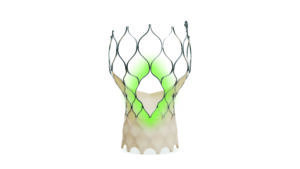
Whilst the Evolut devices have shown a positive performance to date, development does not stand still. Already launched in the USA and now reaching European shores following its recent CE mark approval is the Evolut FX+, the newest addition to the Evolut family. Evolut FX+ offers three larger coronary access windows through a modified diamond-shaped cell design, which are four times larger than regular cells of the Evolut TAVI system. This provides increased space for catheter manoeuvrability to facilitate access to coronary arteries of varying patient anatomies, whilst also designed to maintain its structural strength and radial force.
“In the context of the shift in the ‘typical’ TAVI patient to those that are younger and lower risk, coronary re-access is essential, and these design features are very welcome and useful in that respect,” says McInerney of this latest innovation.
EvolutTM FX+ is indicated for symptomatic severe aortic stenosis adult patients across all risk categories (extreme, high, intermediate, and low) in the European Union and is also indicated for symptomatic severe aortic stenosis patients across all risk categories in the USA.













