Early outcomes following the use of a novel balloon-expandable transcatheter aortic valve implantation (TAVI) device, compared in a randomised trial against two commercially available TAVI valves, have been shared for the first time at EuroPCR 2024 (13–17 May, Paris, France).
Results of the LANDMARK multicentre trial comparing the use of the MyVal (Meril) TAVI valve against the Edwards Lifesciences Sapien, Sapien XT or Sapien 3 valves, or the supra-annular Corevalve, Evolut-R or Evolut Pro valves from Medtronic, in 768 patients with severe aortic stenosis, were presented by Patrick Serruys (National University of Ireland, Galway, Ireland).
A differentiating feature of the valve is its availability in a wider range of prosthesis sizes than the comparator valves, Serruys detailed, with “intermediate” valve sizing making it possible for physicians to avoid over- or under-sizing the prosthesis based on patient anatomy. As many as 48.1% of the valves used in the LANDMARK trial used the intermediate sizing.
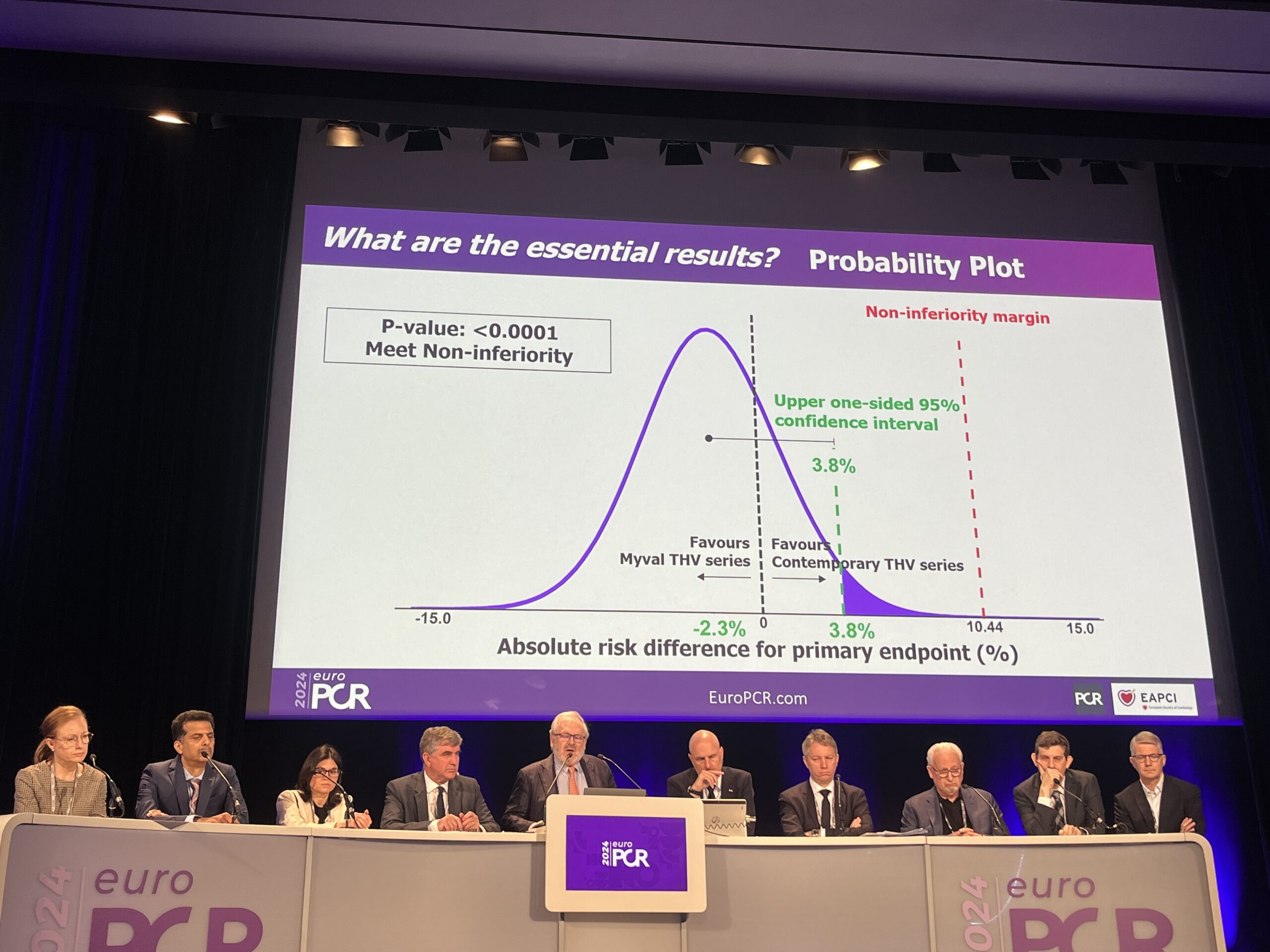
Results of the study showed that the performance of the Myval device in terms of safety and effectiveness was non-inferior to the contemporary devices for the occurrence of a primary composite endpoint of death, stroke, major bleeding, acute kidney injury, major vascular complications, moderate or severe valve regurgitation, and the need for new permanent pacemaker implantation (24.7% vs. 27%).
Secondary endpoints including technical success at exit from procedure room, device success at 30 days and early safety at 30 days were comparable in both the groups.
“Myval THV [transcatheter heart valve] series are novel next-gen THV devices; non-inferior to the Sapien and Evolut THV series,” Serruys was quoted as saying in a press release issued by Meril following the presentation of the results at EuroPCR. “Myval THV series have a unique size matrix incorporating conventional, intermediate and extra-large diameters with increasing diameter steps of 1.5mm that match and fit precisely the multi slice computed tomography (CT) scan-defined aortic annulus area of each individual patient, as a result, provides a superior effective orifice area on echocardiography, which may impact on durability and long-term clinical outcomes.”
“The LANDMARK trial showed that the Myval THV series performed as safe and effective as contemporary THV series,” the trial’s global principal investigator, Andreas Baumbach (Queen Mary University of London, London, UK) was quoted as saying. “It is a valve made for everyday clinical practice and an all-comers population. The special feature of intermediate diameters allows for more accurate sizing, which has the potential to translate into improved long-term outcomes. Our patients will be followed up for 10 years and it will be interesting to see the long-term results in the three treatment arms.”










