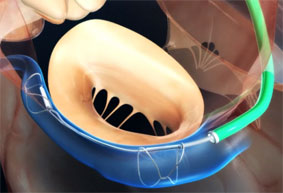
Cardiac Dimensions has announced the launch of the US pivotal study the EMPOWER trial, evaluating the Carillon mitral contour system for the treatment of heart failure patients with early-stage functional mitral regurgitation (FMR).
This blinded, randomised trial will compare the Carillon treatment to a sham-controlled group treated with optimal medical management according to established heart failure guidelines. During development of the trial design, Cardiac Dimensions analysed an extensive amount of data from more than 250 study patients previously enrolled in over four similar trials. Based on these analyses, the device safety profile, and the company’s recent publications, the trial will focus on this extremely large patient population with limited other therapeutic alternatives.
Consistent with prior trials, Cardiac Dimensions will use third-party monitoring, core lab review and external safety reviews, which will produce the most scientifically rigorous clinical data to date in the investigation of heart failure patients with FMR. The combination of quality data, along with the trial being the only blinded, randomised, sham-controlled study performed in the mitral valve space, will make the EMPOWER trial a landmark clinical study in the area of heart failure.
“The launch of our US study represents a significant milestone for the company and big step forward to being able to offer the Carillon therapy to a patient population in need,” said Rick Wypych, Cardiac Dimensions president and chief executive officer. “It is interesting that despite just announcing the trial, we are already seeing overwhelming interest from our sites and the physician community, which is a clear testament to the need for additional device therapies for heart failure patients with early-stage FMR.”
The international trial is expected to randomise 300 patients at up to 75 sites. The study has primary safety and efficacy endpoints at 12 months and will follow the randomised patients out to five years to document long-term safety and clinical status.
“I am very excited to launch the EMPOWER trial,” said Samir Kapadia, interventional cardiologist, professor of medicine and chairman of the Department of Cardiovascular Medicine at Cleveland Clinic, Cleveland, USA and the national principal investigator of the EMPOWER trial. “This will be the first time where we are able to study early intervention with a device in heart failure. The Carillon device will be used to treat heart failure patients with mild and moderate functional mitral regurgitation. This is an extremely large patient population that is currently not being studied by other novel therapies. It will be phenomenal to study the efficacy of the Carillon device in the EMPOWER trial—a rigorously designed, randomised, double blinded, and sham-controlled multicentre clinical trial.”